Spectroscopy
Spectroscopy is the study of energy states of matter, typically through the absorption or emission of electromagnetic radiation or electrons. Spectroscopic instruments at the CCMR can be broadly categorized into optical spectroscopy or vibrational spectroscopy, with electron and X-ray spectroscopy being subsets of optical spectroscopy.
Optical spectroscopy of ultraviolet - visible - near infrared is used to characterize materials based on electronic transitions.
Transmission and reflection spectroscopy can be used to quantify the color of fabric or powder, measure the concentration of dye in a solvent, or determine the bandgap of a semiconductor. Fluorescence spectroscopy probes information about combined vibrational and electronic transitions in a material. Fluorescent lifetime measurements characterize the stability of electronically excited states. Circular dichroism studies the interaction of chiral molecules (those without mirror symmetry) with circularly polarized light, often for the study of protein folding.
Vibrational spectroscopy at the CCMR falls into two general categories. Fourier transform infrared spectroscopy (FT-IR) uses photons with energy equal to the vibrational energy of the molecules being studied. Since it interacts with bonds with inherent dipole moments, it is an excellent method for characterizing organics and polymers. Raman spectroscopy uses higher energy photons (visible light or near IR) and measures the energy spectrum of the small fraction of light which loses energy to vibrational modes of a material. Because Raman scattering is dependent on the polarizability of a bond, most vibrational modes produce either an FT-IR or a Raman signal.
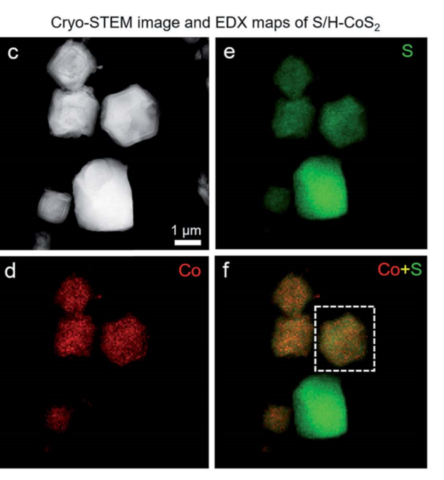
Scanning electron microscopes (SEMs) include energy dispersive X-ray fluorescence spectrometers (EDX) to allow them to map the elemental composition of samples. The primary electron beam in a SEM may kick out core electrons from atoms in a sample, creating vacancies in the electronic structure. Relaxation to the electronic ground state may involve emission of an X-ray photon with an energy specific to that element.
X-ray photoelectron spectroscopy (XPS) uses an X-ray (or UV) source to excite a sample and measures the energy of electrons emitted. High energy resolution provides chemical bonding and oxidation state information in addition to elemental identification.